Driving Digital Transformation
to Unlock Manufacturing Efficiency
Enabling Operational Excellence for Medical Device, Diagnostic, Biotech & Pharmaceutical Manufacturers, and driving digital transformation to unlock manufacturing efficiency.
OUR SOLUTIONS
Let us be your preferred technology partner for
Medical Device, Diagnostic, Biotech & Pharmaceutical Manufacturing.
Our connected manufacturing™ solutions provide real-time data analytics and insights that help to optimise production processes and improve overall efficiency.
As ISO 27001 and ISO 9001 certified manufacturing technology experts, we understand the unique challenges Medical Device, Diagnostic, Biotech, Life Sciences, and Pharmaceutical Manufacturers manufacturers face. Our solutions are designed to address these challenges head-on and ensure that our clients remain compliant with the latest industry standards and regulations.

Digital Manufacturing Cloud
FOR MEDICAL DEVICE AND DIAGNOSTICS MANUFACTURERS
Siemens Optcenter Execution is an advanced digital manufacturing solution that empowers healthcare manufacturers to optimize their production process and improve quality.
Seabrook’s team of manufacturing technology experts is dedicated to providing cutting-edge solutions that meet the needs of the life sciences manufacturing industry.
GxP Compliant eDHR
FOR MEDICAL DEVICE, DIAGNOSTICS, AND BIOTECH MANUFACTURERS
Not ready for a comprehensive MES software transition for your manufacturing processes? Seabrook provides a GxP Compliant eDHR software for life science manufacturers that an iterative, low risk, digital data collection approach that provides immediate value through rapid implementation.

WHAT WE DELIVER
Deploy a Manufacturing Execution System with
Seabrook Technology Group
Our manufacturing technology experts work with clients to identify the unique challenges they face during their manufacturing processes and provide tailored solutions to address those challenges.
We take pride in our ability to customise paperless MES software manufacturing solutions that fit the specific needs of our clients.
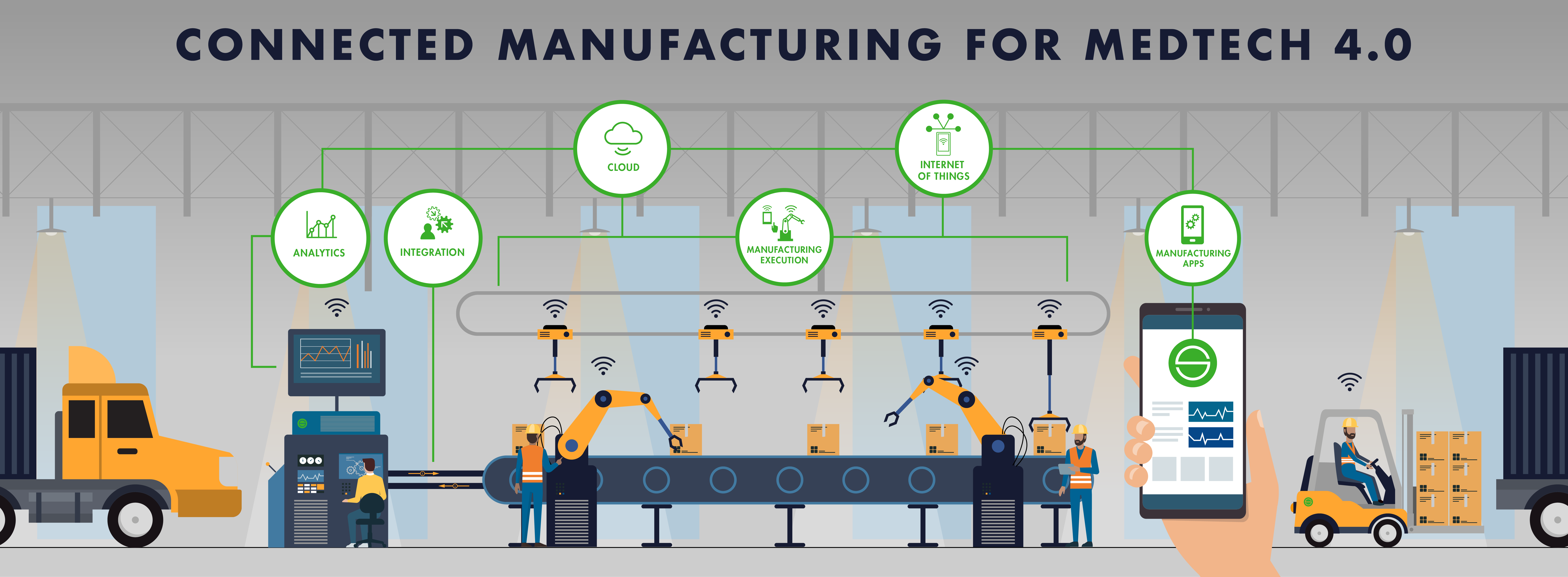
Connected Manufacturing™
Based on industry best practices, our aspiration is to lead Healthcare Manufacturer companies on a Connected Manufacturing™ revolution – bringing a fully integrated approach to manufacturing operations management and embracing Industry 4.0.

MES Consulting Services (Agnostic)
We offer a variety of MES services including Strategic & Site MES Deployment, MES Vendor Selection, Validation and Testing Strategy, and more.
MES Consulting Services (Siemens)
As a Certified Siemens Expert Solution Partner, we provide a wide variety of support services for Siemens Opcenter Execution, including deployment, integration, upgrades, and more.
What Do Our Clients Say…
“The template Seabrook generated to plan for our upgrade activity was very effective and comprehensive, which helped us to systematically work the issues.”
“We would never have been able to do this on our own without Seabrook.”
“Seabrook was a great help in doing the analysis and creating a report that allowed us to execute our software selection and implement our MES through a private cloud-based network.”
WANT TO WORK WITH US?
Find out more about what differentiates
us from our competitors.
TRUSTED BY INDUSTRY EXPERTS
Some of the companies we are proud to support as a leader in Medical Device, Diagnostic, Biotech & Pharmaceutical Manufacturers.
CAREERS
We Are Hiring!
We are a team of manufacturing technology experts and industry thought leaders, and we are looking for talented professionals to join our team.


GET IN TOUCH
Let’s build a better future together
Have a question? Need answers? Let’s talk!























